Unicellular algae form a powerful biotechnological warehouse for production of chemical compounds. However, algae commercial potential can be fully revealed only after deepening the understanding of their fundamental processes.
Triacylglycerols (TAGs) are lipids that can be used as theprecursors in biodiesel production. Model green alga, Chlamydomonas reinhardtii, accumulate TAGs in stress conditions, like nitrogen deprivation. TAGs accumulation is reversible and chromatin-based, features that make this process a potential subject for stress priming - a phenomenon that was not explored in algae; yet it offers important opportunity to increase stress-induced TAGs bioproduction.
Here, we are proposing a non-hardware project to establish Chlamydomonas growth protocols in stress priming setup. We will optimize priming conditions and measure TAGs content through Nile Red staining with fluorescence microscopy analysis.
The aim of the project is to provide proof-of-principle evidence for stress priming in algae, which will ultimately lead to potentially improved yields of biotech-relevant compounds. The results will serve as a resource for industrial and scientific community.
ProposalThe problem
Unicellular algae form a powerful biotechnological warehouse for production of chemical compounds [1]. However, the current yields of many of such compounds are below threshold for commercial viability. Full bioproduction potential in algae can be achieved through optimization and deepened understanding of their relevant fundamentalprocesses.
Model green alga Chlamydomonasreinhardtii can serve as the natural source of triacylglycerols (TAGs) [2], that in turn are used as the precursors of biodiesel. TAGs accumulation in algae remains still not fully understood and current experimental setups lead to yields below potentially achievable.
Solution
In order to establish a way to obtain higher TAGs yields, we are proposing a novel, stress priming-based, experimental setup for model unicellular green alga Chlamydomonas reinhardtii. TAGs accumulation in Chlamydomonas is induced by adverse environmental conditions, i.e. nitrogen deprivation in the media, and serves as a response mechanism against the stress. Such stress response is reversible and depends on the chromatin and epigenetic changes of relevant genes [3, 4]. Reversible, chromatin-related stress responses in the other systems are frequently connected with stress priming phenomenon, where initial stress-induced changes can be memorized and amplified upon further stress exposure [5]. Priming experiments offer an important opportunity to potentially increase TAGs yields in labor-effective way and without specific instrumentation.
Biological systems
The biological system of the project is the model unicellular green alga, Chlamydomonas reinhardtii. Commonly used cc-125 wildtype strain of Chlamydomonas will be grown as liquid culture in Erlenmeyer flasks in growth chambers at 25 °C with agitation at 180 rpm under light irradiation at 100 µE [3]. Growth medium will be based on the TAP buffer with or without nitrogen salts [6] for nitrogen-rich (nitrogen-control) and nitrogen-depleted media, respectively.
Project implementation
1) Set up stress priming growth conditions [Fig.1], including the selection of proper harvesting timepoints and preservation of harvested material (formaldehyde fixation)
2) Implement and optimize TAGs staining protocol using Nile Red dye [7]
3) Acquire images using SP5 confocal microscope
4) Quantify dye intensity, normalize to cell size and extrapolate to TAGs accumulation strength. Nile Red fluorescence is tightly correlated with TAGs content [4]
5) Disclose protocols and reports for the outreach to the community in text and graphical summary
Outcomes and benefits
Proposed project will provide the proof of concept for stress priming phenomenon in algae. It will lead to optimization of TAGs staining and stress priming growth for algal cultures. All protocols will be open for the public at the end of the project. The outcomes are meant to help green biotech industry and relevant scientific community to establish their stress priming growth protocols.
References:
1. Brodie J, Chan CX, De Clerck O, Cock JM, Coelho SM, Gachon C, et al. The Algal Revolution. Trends in Plant Science. 2017;22:726–38.
2. Merchant SS, KropatJ, Liu B, Shaw J, Warakanont J. TAG, You’re it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Current Opinion in Biotechnology. 2012;23:352–63.
3. Ngan CY, Wong C-H, Choi C, Yoshinaga Y, Louie K, Jia J, et al. Lineage-specific chromatin signatures reveal a regulator of lipid metabolism in microalgae. Nat Plants. 2015;1 July:1–11. doi:10.1038/NPLANTS.2015.107.
4. Cagnon C, Mirabella B, Nguyen HM, Beyly-Adriano A, Bouvet S, Cuiné S, et al. Development of a forward genetic screen to isolate oil mutants in the green microalga Chlamydomonas reinhardtii. Biotechnol Biofuels. 2013.
5. Mozgova I, Mikulski P, Pecinka A, Farrona S. Epigenetic Mechanisms of Abiotic Stress Response and Memory in Plants - Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications: Transcriptional Regulation and Chromatin Remodelling in Plants. In: Alvarez-Venegas R, De-la-Peña C, Casas-Mollano JA, editors. Cham: Springer International Publishing; 2019. p.1–64. doi:10.1007/978-3-030-14760-0_1.
6. Boyle NR, Page MD, Liu B, Blaby IK, Casero D, Kropat J, et al. Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J Biol Chem. 2012;287:15811–25.
7. Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U. Algal lipid bodies: Stress induction, purification, and biochemical characterization in wild-type and starchless chlamydomonas reinhardtii. Eukaryot Cell. 2009;8:1856–68.
Project developmentTests & optimization- Light& temperature
We firstly optimized conditions for growing C. reinhardtii in our growth cabinets. We tested light intensity in different set-ups and checked temperature fluctuations.
We measured light intensity on different positions on the shelf and with various number of bulbs switched on/off. We selected combination that fitted to the published standard light regime [Fig.2]. For C. reinhardtii growth we set constant light condition.
We set constant 25°C as the growth temperature. The temperature was kept at the proper level without fluctuations [Fig.3].
- Growth speed
Next, we tested growth of C. reinhardtii in conditions above, in low-volume test. To asses growth speed, we measured optical density (OD600) for cells grown in nitrogen-control (nitrogen-rich) and nitrogen-depleted media [Fig.4]. Our result indicated that the recovery period (between ZT2 and ZT3 [Fig.1]) should be extended to 2 days (original proposal had 1 day-long recovery).
- Fixation & staining
Our set-up involved histochemistry using TAGs staining with Nile Red dye. Firstly, we optimized chemical fixation of cells - we used 3% (v/v) formaldehyde (FA) on 1 ml culture aliquots, incubated for 20 min on ice, spun at 3000 rpm for 5 min and washed twice with phosphate saline buffer (PBS). Final cell pellet was resuspended in 20-50 uL PBS, depending on the OD600. 6-7 μL of final solution was spread onto microscopy slides, air dried and refrigerated until staining. Staining was done with 5 μg/mL in 0.1 % acetone for 15 min in dark.
- Microscopy
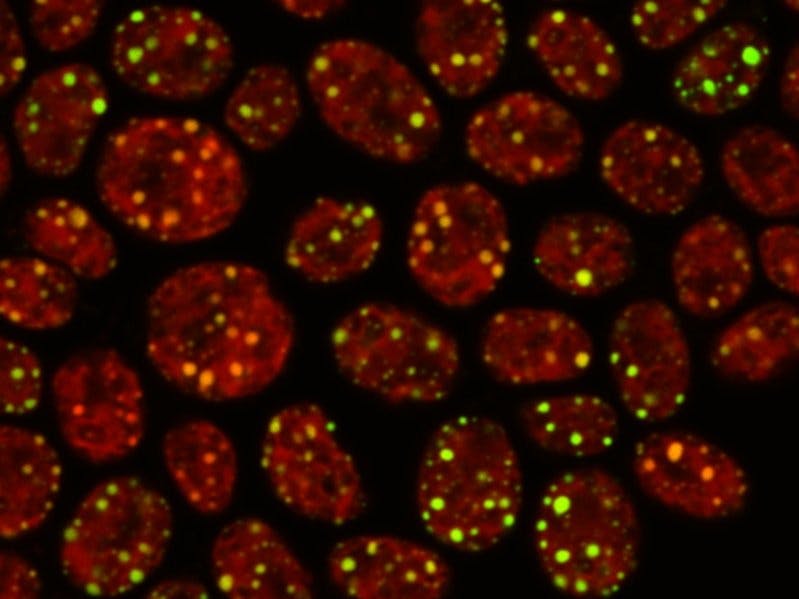
We set image acquisition parameters to maximize signal-to-noise ratio and decreased bleed-through from chloroplast autofluorescence by separating excitation/emission (Ex/Em) wavelength ranges into 2 channels: Ex488/Em520-600 and Ex633/Em635-700. Very low, background-level fluorescence in the negative controls (mock-treated nitrogen-starved cells and Nile Red-stained nitrogen-control cells) validated the specificity of our approach. As expected, nitrogen-starved cells showed high fluorescence signal, in contrast to the nitrogen-control cells that were largely devoid of specific signal [Fig.5]. For intensity quantifications, we used Ex488/Em520-600 channel, as it showed more specific signal to Nile Red and no crosstalk with autofluorescence.
We acquired Z-stacks of cells to capture whole Nile Red signal in 3D. Our step size (z-distance between slices) was kept at 0.55-0.59 μm and line averaging at 2. We set scan speed at 400 Hz, pinhole size at 1 Airy unit (AU) and laser power at 16%.
- Image analysis
Image analysis was performed in Fiji/ImageJ. Following steps were undertaken: 1) Z-stacks were converted to single image by slice summing in Z-projection; 2) cells were automatically segmented with minimum error threshold (with manual fine-tuning) to create masks; 3) masks were used as regions-of-interest (ROIs) to outline cell borders; 4) mean fluorescence intensity (grey value) was quantified inside ROIs [Fig.6]. To normalize technical differences in fluorescence intensities between different frames/samples, we quantified background fluorescence outside of cells, averaged it and subtracted from the specific signal from within the cells.
We set the experiment according to the original design [Fig.1] for each sample variant, N+/- and N-/-. We harvested cells at all timepoints and proceeded to microscopy with sample ZT4 N-/- (N-/- sample subjected to repeated stress) and its minimal set of controls: ZT2 N-/- (N-/- sample subjected single, 1st stress) and ZT4 N+/- (control N+/- sample subjected to single, 2nd stress). We quantified 154-300 cells per sample to ensure statistical power of the analysis.
Our intensity measurements showed that cells subjected to repeated stress (N-/- variant) accumulate less TAGs under 2nd stress exposure (ZT4) comparing to the same samples under 1st stress (ZT2 N-/-) or the other single-stress control (ZT4 N+/-) [Fig.7].
Our results are unexpected, but explainable. Firstly, they highlight that stress memory exists in model green alga as we can see a clear difference in TAGs accumulation between single-stress and repeated-stress cells. Secondly, TAGs downregulation in repeated-stress variant indicate that cells respond to 2nd stress exposure with pathways antagonistic to TAGs accumulation. Answering what those pathways are opens new avenues to study mechanisms of stress memory and offers an exciting subject for open innovation in green biotechnology.
Given the importance of the findings above, we sought to repeat the experiment to make the data more reliable. We observed slight differences in culture growth rates between biological replicates in the first experiment, what prompted to adjust passage frequency accordingly in the repeated experiment. We proceeded with the same steps as before, except decreasing growth duration in the recovery period between ZT2 and ZT4 to 24 hours.
Repeated experiment [Fig.8] showed the results consistent with the 1st try (!), proved robustness of our technical setup and increased reliability of the conclusion that stress memory exists in model green alga.
- Lipid profiling (preliminary data)
To qualitatively assess the lipid composition of the accumulated TAGs and complement the confocal microscopy measurements, we performed a gass-chromatography-mass-spectrometry (GC-MS) lipidomics analysis. For this, the time-course samples were extracted using chloroform/methanol (2:1). The extracted lipids underwent transesterification to generate volatile methylester fatty acids, which were subsequently analysed by GC-MS. The observed MS fragmentation patterns and retention times for each peak were then compared with standard libraries for the identification of the most abundant fatty acids in the samples. We performed GC-MS for 1st experiment (samples from 2nd experiment are under analysis) [Fig.9, 10].
Our results suggested following main points:
1) the qualitative profile of lipid types did not show substantial differences between single- and double-stress samples, despite different quantitative lipid accumulation that we saw through histochemistry;
2) any of the stressed samples, double or single, showed reduced number of peaks, comparing to the non-stressed control (ZT2 N+/-), suggesting that nitrogen deprivation causes a reduction of lipid profile complexity;
3) under stress conditions, saturated lipids (mainly palmitic acid) seem to increase their abundance ratio in respect to unsaturated lipids
We observed also some variation between biological replicates and sought to repeat GC-MS experiment to increase the reliability of the findings.
Project Title:
Stress priming for improved production of biotech-relevant compound in green alga
Summary
Triacylglycerols (TAGs) are lipids that can be used as the precursors in biodiesel production to provide much needed clean energy source. Model green alga, Chlamydomonas reinhardtii, accumulate TAGs under stress conditions, like nitrogen deprivation. The aim of our project was to hyperinduce TAGs production by stress priming, a phenomenon where initial stress-induced changes can be memorized and amplified upon further stress exposure.
In the project, we successfully set up optimal conditions and growth duration for algal liquid cultures by measuring culture optical density, light intensity and temperature. We optimized protocols for cell fixation, TAGs staining, microscopy and image analysis. Finally, we successfully quantified lipid fluorescence intensity (based on Nile Red staining) in main samples and various controls.
Our final measurements came with a surprise and showed that algal cells have decreased TAGs levels upon repeated stress instead of a hyperinduction. As recurrently stressed cells did not exhibit growth retardation during recovery period, our results indicate that C. reinhardtii can indeed be primed and contain stress memory mechanisms. However, its response to repeated stress exposure is based on the pathways antagonistic to lipid accumulation.
Our results provide a proof-of-principle for existence of stress priming in model green alga and offer an exciting subject for open innovation in green biotechnology.
Report and outcomes
Test
Firstly we optimized conditions for growing C. reinhardtii in our growth cabinets. We tested light intensity in different set-ups and checked temperature fluctuations. We assessed growth speed by measuring optical density (OD600) in small-volume test liquid culture. Growth speed measurements indicated that recovery periods (between ZT2 and ZT3 [Fig.1]) should be extended to 2 days.
Our set-up involves histochemistry using TAGs staining with Nile Red dye. We optimized chemical fixation of cells and their staining on test samples. As expected, nitrogen-starved cells showed high fluorescence signal under microscope, in contrast to nitrogen-control cells that were largely devoid of specific signal. We set image acquisition parameters to maximize signal-to-noise ratio and decreased bleed-through from chloroplast autofluorescence by separating excitation/emission wavelength ranges into 2 channels (similarly to PMID:24349166). Very low, background-level fluorescence in the negative controls (mock-treated nitrogen-starved cells and Nile Red-stained nitrogen-control cells) validated the specificity of ourapproach.
Full experiment-procedure
We set the full experiment based on the design and harvesting timepoints written in the original proposal (with extended recovery period). Cells were chemically fixed immediately after harvesting, spread onto microscopy slides and refrigerated until the end of growth timecourse. At each timepoint we also measured culture OD in triplicate. We proceeded to microscopy with main sample (ZT4 N-/-) and its minimal set of controls (ZT2 N-/-) and (ZT4 N+/-) [Fig.1]. We used the same microscopy parameters to all the samples.
We acquired Z-stacks of cells and subjected them to image post-processing and fluorescence quantification in Fiji/Image J. Following steps were undertaken: 1) Z-stacks were converted to single image byslice summing in Z-projection; 2) cells were automatically segmented with minimum error threshold to create masks; 3) masks were used as regions-of-interest (ROIs) to outline cell borders; 4) mean fluorescence intensity was quantified inside ROIs. To normalize technical differences in fluorescence intensities between different frames/samples, we quantified background fluorescence outside of cells, averaged it and subtracted from the specific signal from within the cells. To ensure statistical power, we quantified intensity of 154-300 cells per sample.
Full experiment-outcomes
Our intensity measurements showed that cells subjected to repeated stress (N-/- variant) accumulate less TAGs under 2nd stress exposure (ZT4) comparing to the same samples under 1st stress (ZT2 N-/-) or the control experiencing single stress (ZT4 N+/-). The fact that both samples with cells treated with single stress only (ZT2 N-/- and ZT4 N+/-) show comparable levels of TAGs accumulation highlights the robustness of our analysis.
Our results are unexpected, but explainable. Firstly, they highlight that stress memory exists in model green alga as we can see a clear difference in TAGs accumulation between single-stress and repeated-stress cells. Secondly, TAGs downregulation in repeated-stress variant indicate that cells respond to 2nd stress exposure with pathways antagonistic to TAGs accumulation. Answering what those pathways are opens new avenues to study mechanisms of stress memory and impacts biofuel production processes for green biotechnology.
Follow on Plans
Histochemistry-based quantification gives information about general level of TAGs in cells. However, the precise composition of the accumulated TAGs can vary depending of the chemical nature (chain length, unsaturation degree) and can be dynamically regulated upon stress treatments. Therefore, we would like to expand our quantitative-data proposal by adding qualitative analysis about different TAGs types.
Our plan is to use gas chromatography–mass spectrometry (GC-MS) as a method of choice. We contacted on-site Metabolomics facility, which can offer us discounted rate for machine usage (normal rate = 40 GBP/hour), making the experiment highly cost-effective. The material for GC-MS runs is available - along microscopy samples, we were harvesting bigger aliquots that would be enough for this method. Furthermore, we will take advantage of GC sample preparation to validate our current histochemistry results - isolated lipids can be stained ex-vivo and quantified in spectrometer.
Given above, we envisage that the analysis can becompleted by the end of August. The summary of the new results and open innovation protocols will be ready for Open Technology Workshop in November. As most of the required materials are already available, follow-on funding will be consumed by GC-MS machine usage.


Comments
Please log in or sign up to comment.