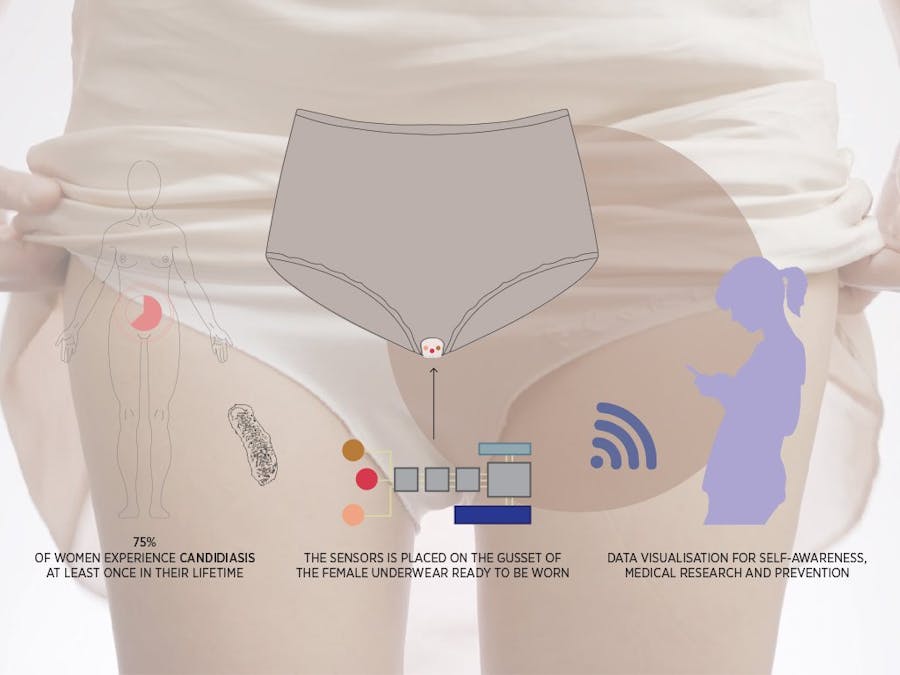
Winner of Biomaker Spirit Award at Cambridge Biomaker Challenge 2018Gynaecological conditions, particularly infections such as Candidal vulvovaginitis (CVV) and Bacterial Vaginosis (BV), are still a significant burden for many women, particularly as these may be recurrent. Societal and cultural stigma and often adoption of unconventional treatment pose a risk of harm to many. This project stems from the idea of developing an inconspicuous, low-cost wearable biosensor aimed at monitoring physiological markers of infection, such as lactate and pH, in vaginal secretions. On one hand, this will provide insight into what normal and abnormal physiology may be for individual women. On the other, we hope this will provide new insight into the underlying biological processes for research purposes. We hope this will enable women to take up a more active role in their healthcare, prompting them to seek medical advice as necessary and ultimately break some of the taboos associated with urogynaecological health.
The goal for this project is to create a wearable, reusable and inexpensive sensing platform for vaginal fluid analysis, see figure 2. Studies have shown that the most important biomarkers in vaginal fluids are pH and lactate [1,2]. Both markers can be simultaneously and selectively detected by our sensors array shown in figure 3. The pH and lactate sensors are respectively based on potentiometric and amperometric sensing. The sensors array is embedded in the gusset of the female underwear and is connected to a flexible printed circuit board(PCB), creating a fully integrated device, capable of signal transduction, conditioning (amplification and filtering), processing and wireless transmission. Both the array and the board are fabricated on flexible plastic substrates, making this device ideal for wearable applications. The entire device is designed for prolonged use,however, the sensors array will have a limited lifetime as the enzymes in the lactate sensor will degrade. Therefore, we have designed the array to be easily removable and replaceable, reducing waste and cost. To further reduce cost, we have used proven manufacturing methods and materials.
The sensors array is largely based on existing wearable sensors [3,4]. These sensors have been validated for sweat, saliva and tears analysis[3,5,6]. The complete fabrication is shown in figure 2. While these previous models have utilised photolithography, which is expensive and complex, the main improvement of our design involves using aerosol jet printing (AJP) to fabricate the electrode and conductive paths. This method is cheaper, quicker and easily adaptable to different design requirements [7]. The electrode and conductive paths will be printed using silver ink on a flexible polyethylene terephthalate(PET) film. The conductive paths will be insulated by printing a layer of polyimide over them.
Project ProgressionThe pH sensor was fabricated first due to its simpler design. The key tasks were: reference electrode fabrication, pH electrode fabrication and sensor testing. All the fabrication were done in the Kar-Narayan Lab in the Department of Materials Science, University of Cambridge.
Reference ElectrodeThe silver electrode and the conductive paths were successfully printed using AJP. The silver electrode was transformed into a Ag/AgCl reference electrode by drop casting a solution of FeCl3. The formation of the reference electrode was confirmed by Raman spectroscopy.
The pH working electrode was fabricated by electrodeposition of polyaniline(PANI) onto the electrode using cyclic voltammetry[14]. Initially, the electrode material was silver, but experimental work showed that no polyaniline was formed on the electrode surface. This was likely caused by the chlorination of the the silver electrode surface during the electrodeposition. Therefore, the electrode material was switched to gold as it is a more stable metal. The deposition of polyaniline on the gold was successful and it was confirmed using Raman spectroscopy.
Sensor Testing
The pH and reference electrode were connected to created a sensor. The sensor performance was evaluated using potentiostat and several pH buffer solutions. The potential between the two electrodes was measured and used to determine pH. The sensor successfully measured changes in pH when pH buffer solution was added.
ALMA AppA user-friendly mobile app was designed to interface the sensor. The sensor will sample the fluid pH at regular intervals, send information via bluetooth to the user's smartphone, and display the data gathered. We are hopeful this will generate awareness and promote health-seeking behaviours where necessary.
AcknowledgementsWe are grateful to SynBio SRI Fund and OpenPlant Fund for the financial support. The team would like to thank Dr. Sohini Kar-Narayan for her support and access to equipment.
References[1]EW Henn, TF Kruger, and TI Siebert. Vaginal discharge reviewed: the adultpre-menopausal female. South African Family Practice, 47(2):30–38, 2005.
[2]O Niklasson, G Skude, and P-A Mrdh. Lactate dehydrogenase and its isoenzymes invaginal fluid in vaginitis/vaginosis cases and in healthy controls.International Journal of STD & AIDS, 14(4):270–273, 2003. PMID: 12716498.
[3]Wei Gao, Sam Emaminejad, Hnin Yin Yin Nyein, Samyuktha Challa, Kevin Chen,Austin Peck, Hossain M. Fahad, Hiroki Ota, Hiroshi Shiraki, Daisuke Kiriya,Der-Hsien Lien, George A. Brooks, Ronald W. Davis, and Ali Javey. Fullyintegrated wearable sensor arrays for multiplexed in situ perspirationanalysis. Nature, 529:509 EP –, Jan 2016.
[4]Hnin Yin Yin Nyein, Wei Gao, Ziba Shahpar, Sam Emaminejad, Samyuktha Challa,Kevin Chen, Hossain M. Fahad, Li-Chia Tai, Hiroki Ota, Ronald W. Davis, and AliJavey. A wearable electrochemical platform for noninvasive simultaneousmonitoring of ca2+ and ph. ACS Nano, 10(7):7216–7224, 2016. PMID: 27380446.
[5]Ming Xing Chu, Kumiko Miyajima, Daishi Takahashi, Takahiro Arakawa, Kenji Sano,Shin ichi Sawada, Hiroyuki Kudo, Yasuhiko Iwasaki, Kazunari Akiyoshi, ManabuMochizuki, and Kohji Mitsubayashi. Soft contact lens biosensor for in situ monitoringof tear glucose as non-invasive blood sugar assessment. Talanta, 83(3):960 –965, 2011.
[6]Mohammad A. Javaid, Ahad S. Ahmed, Robert Durand, and Simon D. Tran. Saliva asa diagnostic tool for oral and systemic diseases. Journal of Oral Biology andCraniofacial Research, 6(1):67 – 76, 2016.
[7]Michael Smith, Yeon Sik Choi, Chess Boughey, and Sohini Kar-Narayan.Controlling and assessing the quality of aerosol jet printed features for largearea and flexible electronics. Flexible and Printed Electronics, 2(1):015004,2017.
















Comments
Please log in or sign up to comment.