Cell-free protein expression system is an efficient tool to synthesize a large range of proteins-of-interest in vitro and study their functionality. The real biological systems are complicated by the massive number of components and highly complex cellular network. Establishing a cell-like environment in vitro allows us to disentangle the basic mechanisms underlying complex cellular network in a well-controlled system.
In this project, we aim to develop an artificial cellular system using microfluidic droplets that allows the in vitro expression of proteins from plants in a compartmentalized cell-like environment. The betalain biosynthetic pathway will be used as a simple model to establish the method. The cell-free protein expression components together with betalain biosynthetic genes will be enclosed in microdroplets to test if an artificial cellular system for plant protein expression can be achieved.
Furthermore, the communication between cells plays a central role in the metabolic pathways of multicellular organisms, yet this pattern remains difficult to reproduce in vitro. To this end, the potential to establish an artificial multicellular system with droplet-to-droplet communication will be explored to mimic metabolic pathways that rely on the communication between cells. These systems would open more opportunities for synthetic biology in plants and allow hypotheses and models on cell communications to be tested in vitro.
Biological systemBetalains are a class of red and yellow pigments, betaxanthins and betacyanins, that only occur in the order Caryophyllales. The betalain biosynthetic pathway involves two to four core enzymes to synthesise betalains from L-tyrosine (Fig. 1). Three cytochromes P450, CYP76AD1, CYP76AD5 and CYP76AD6, are all capable to catalyse the first step to convert L-tyrosine to L-3, 4-dihydroxyphenylalanine (L-DOPA). One of the P450, CYP76AD1, subsequently converts L-DOPA to cyclo-DOPA. 4, 5 DOPA dioxygenase (DODA) converts L-DOPA to betalamic acid, which can spontaneously react with cyclo-DOPA to form unglycosylated betacyanins, betanidins, or with various amino acids to form betaxanthins. Cyclo-DOPA can be glucosylated by cyclo-DOPA 5-O glucosyltransferase (cDOPA5GT) to form cDOPA 5-O-glucoside, which spontaneously reacts with betalamic acids to produce betacyanins. The final products, betaxanthins and betacyanins, absorb light in the visible spectrum and so are easy to detect and quantify. Betaxanthins also emit green fluorescence.
The heterologous production of betacyanin in Nicotianabenthamiana has shown to produce visible red pigmentation. Given the easy detection of final products of this pathway, we are hoping to use this pathway as a model to explore to what extent microdroplets can be used to mimic real metabolic pathways from plants. To start with, we are trying to introduce CYP76AD1 and DOPA to the cell-free microdroplet system and detect the red pigmentation of microdroplets as a result of betanidins production. Furthermore, we would like to compartmentalise different steps of the pathway in different droplets to mimic multicellular pathways, and evaluate the efficiency and reproducibility of this droplet-to-droplet communication.
Workflow
1. Test in vitro protein expression with the commercial kit in microdroplets using GFP.
a) In vitro expression of GFP in ep tubes using cell-free plant protein expression kits.
b) In vitro expression of GFP in microdroplets.
2. Express betalain biosynthetic pathway enzymes and evaluate enzyme activities.
a) In vitro expression of betalain biosynthetic enzymes in ep tubes and analysis of the enzyme activities.
b) In vitro expression of betalain biosynthetic enzymes in microdroplets and measurement of betalain production.
3. Explore the methods to control the metabolite flow between microdroplets to mimic multicellular pathways.
MaterialGeneral material for plasmid preparation and in vitro protein expression
TNT® SP6 High-Yield Wheat Germ Protein Expression System (Promega)
Phusion® High-Fidelity DNA Polymerase and its corresponding buffers
L-tyrosine (Sigma-Aldrich)
L-DOPA (Sigma-Aldrich)
Microfluidics device
The microfluidic device was fabricated via soft lithography by pouring poly(dimethylsiloxane) (PDMS) along with crosslinker (Sylgard 184 elastomer kit, Dow Corning, Midland, MI, USA; pre-polymer: crosslinker = 10: 1) onto a silicon wafer patterned with SU-8 photoresist (Ref: [1] Qin D, Xia Y, Whitesides GM. Rapid prototyping of complex structures with feature sizes larger than 20 μm. Adv Mater. 1996; 917–919. [2] Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal Chem. 1998;70: 4974–4984. pmid:21644679). See also https://www.protocols.io/view/pdms-microfluidic-device-fabrication-ftybnpw
Liquid chromatography–mass spectrometry (LC-MS) device
Acquity UPLC H-Class system
CORTECS C18+ column (1.6 µm)
Waters LCT Premier Mass Spectrometer
ExperimentsPreparation of DNA templates for in vitro transcription and translation (IVTT)
SP6 RNA polymerase promoter (TATTTAGGTGACACTATAGAACAGACCACC) was used to drive the cell-free protein expression with TNT® SP6 High-Yield Wheat Germ Protein Expression System (TNT system). The SP6 promoter sequence was introduced to the upstream of the cDNA sequence of gene-of-interest by PCR, where the 5' primer carries the complete sequence of the SP6 promoter and part of sequence from the gene-of-interest, and the 3' primer consists of the 3' end of the gene-of-interest (Fig. 2). The PCR products were directly used for the IVTT experiments without further purification.
IVTT of GFP in tubes
The DNA templates of SP6::GFP were generated from the PCR reactions described above. 25 μl TNT® SP6 High-Yield Wheat Germ Master Mix was mixed with 8 μl PCR products and diluted up to 50 μl with nuclease-free water. The reaction solution was incubated at 25 °C from 2 hours to overnight. A reaction mix with water added in place of PCR products was used as control. At 2.5h, 5h, 8h and 26h after incubation, 10 μl of each reaction solution was transferred into a 96-well plate and diluted to 50 μl with water. Fluorescence was measured at excitation/emission wavelengths = 470±15/515±20 nm with CLARIOstar® Plus and the GFP fluorescence signals were blanked with control. Reaction solution that contained GFP PCR products shown strong fluorescence after 2 hours and the signal lasted at least up to 26 hours. The fluorescence from GFP accumulation is shown in Fig. 3.
IVTT of GFP in microdroplets
Microdroplets containing the DNA templates of SP6:GFP and TNT® SP6 High-Yield Wheat Germ Master Mix were generated by a flow-focusing microfluidic device. To generate water-in-oil microdroplets, three different liquids were injected into a microfluidic device by three syringe pumps (PHD, Harvard Apparatus) with controlled flow rates (Fig. 4A). One aqueous phase for inlet 1 was DNA templates of SP6:GFP and another aqueous phase for inlet 1 was TNT® SP6 High-Yield Wheat Germ Master Mix. 3MTM NovecTM 7500 perfluorinated oil containing a 1% Pico-Surf™ 1 surfactant was used as the continuous phase for inlet 3.
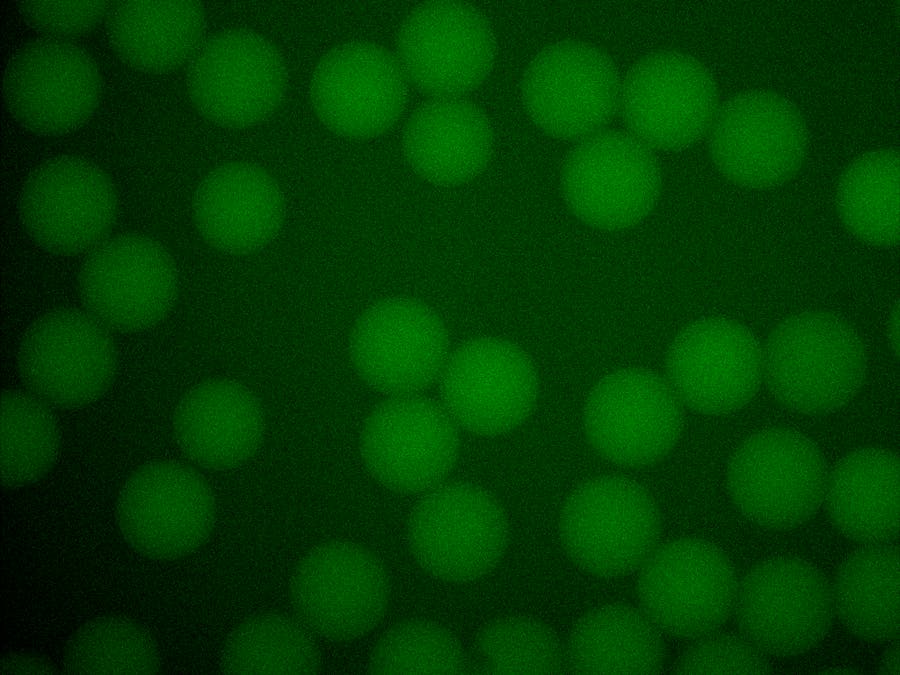
The continuous phase (inlet 3) and both discontinuous phase solutions (inlet 1 and 2) were separately loaded into 500 μL syringes before connecting to the microfluidic chip. Syringes with needles were mounted on syringe pumps and fitted with polyethylene tubing, while the other end of the tubing was inserted into the appropriate inlets of a microfluidic chip. Microdroplets formation was initiated by pumping 3MTM NovecTM 7500 perfluorinated oil into the device at the rate of 200 μL/h, following by pumping of SP6:GFP and TNT® SP6 High-Yield Wheat Germ Master Mix at the same speed of 60 μL/h. Upon generation, the microdroplets were collected in a 500 μL centrifuge tube. To image the microdroplets, they were transferred to a microscope slide under an inverted fluorescence microscope (Olympus IX81) attached with a camera (Andor Technology EMCCD iXonEM+ DU 897). Strong GFP signal was observed 3 hours after microdroplet generation (Fig. 4B).
Then it's time to play with some more interesting enzymes
The in vitro expressions of SP6:GFP in tubes and in microdroplets have both given consistent and reliable results. Our next step is to see if we can build the betalains biosynthetic pathway using this system.
To start with, cytochrome P450 76AD1 (76AD1) and 4, 5 DOPA dioxygenase (DODAα) from beta vulgaris, which are enzymes capable to catalyse the conversion from L-tyrosine to the red unglycosylated pigment betanidins (see Fig. 1), were tested in tubes and microdroplets. In order to track the expression of enzymes, we tagged the enzymes with different fluorescent proteins and tested different combinations to optimise the fluorescence signal.
First, C-terminal fusions of eGFP and mCherry to each enzyme were tested as we thought it could serve as a good proof of the transcription and translation of the entire DNA template compared to N-terminal fusions. For the measurement of GFP signals, fluorescence was measured at excitation/emission wavelengths = 470±15/515±20 nm with a gain setting of 2000 using CLARIOstar® Plus. For the measurement of mCherry signals, fluorescence was measured at excitation/emission wavelengths = 570±15/620±20 nm with a gain setting of 3000 using CLARIOstar® Plus. Three measurements were made on each sample at each time point.
For the test of 76AD1::mCherry and DODA::GFP, 30 μl TNT® SP6 High-Yield Wheat Germ Master Mix was mixed with 7 μl PCR products and diluted up to 50 μl with nuclease-free water. SP6:GFP and SP6:mCherry were used as positive controls (Fig. 5A-B). For the test of 76AD1::GFP and DODA::mCherry, the condition was slightly changed to reduce the consumption of cell-free kits, where 12 μl TNT® SP6 High-Yield Wheat Germ Master Mix was mixed with 7 μl PCR products and diluted up to 40 μl with nuclease-free water (Fig. 5C-D). This setting was used for the rest of the cell-free reactions unless stated otherwise. In both experiments, the mixture of cell-free components with water instead of template DNA was used as the blank control. 10 μl of the reaction solution was diluted to 50 μl with water when loading onto 96-well plates prior to measurement.
Fluorescent protein fusions showed much weaker signals compared to pure fluorescent proteins, and the combinations 76AD1 ::GFP and DODA::GFP did not show any significant signal. We adjusted the position of the GFP on DODA and tested them again in tubes and also in microdroplets this time (Fig. 6A-B and 6C-F respectively).
However, the 76AD1::mCherry which initially showed some fluorescence from 96-well pates failed to work in the microdroplet environment, so we had to design a new N-terminal fluorescent protein fusion for enzyme 76AD1 (Fig. 7).
Two positive fluorescent protein fusions, mCherry::76AD1 and GFP::DODA, were then co-incubated with cell-free protein expression components in microdroplets, which showed both GFP and mCherry signals after overnight incubation as proof of enzyme expressions (Fig. 8).
One of the lessons we have learnt with these experiments so far is that in the cell-free environment the fluorescent protein fusions might be more picky about the relative position of fluorescent proteins and tagged proteins compared to normal cellular systems.
In the next step, mCherry::76AD1 and GFP::DODA were incubated separately in cell-free reaction solutions and fluorescence was measured 5 hours after incubation to confirm the expression of corresponding enzymes. Then these two components were mixed together and incubated overnight with or without the addition of 0.25mM L-tyrosine. The light absorbance at 540 nm was measured to detect the production of betanidins (Fig. 9).
Here we were not able to detect any visible colour or any change of light absorbance in the reaction solutions. In fact, the variations of light absorbance values among different samples and among different measurements of one sample were so large that no reliable conclusion can be drawn from comparing these values. We suspected that these variations mainly derived from the pipetting errors of TNT® SP6 High-Yield Wheat Germ Master Mix, which had a significant background on the light absorbance spectrum itself, at each measurement because of the existence of surfactants in the buffer.
Difficult to track the change of OD? Try betaxanthin!
So even though we could track the expression of proteins with fluorescent proteins, the background of cell-free kit on the light absorbance spectrum is very high and strongly interfering with the detection of betanidins. Furthermore, the fact that both CYP76AD1 and DODA could take L-DOPA as substrates added some complexity to the system, so we decided to replace CYP76AD1 with CYP76AD6 and introduce the biosynthesis of betaxanthin into microdroplets. One advantage of betaxanthin over betacyanin is that the former one also emits green fluorescence, which is easier to measure than light absorbance according to our previous experiments as the cell-free kit itself does not have a huge background.
The fluorescence of betaxanthin has similar excitation and emission wavelengths as GFP, therefore the same setting was used to measure betaxanthin. In order to obtain a clearer background for the detection of betaxanthin, no fluorescent protein fusion was used to track the expression of enzymes. SP6:76AD6 SP6:DODA (3.5 μl of each) were mixed with 12 μl TNT® SP6 High-Yield Wheat Germ Master Mix and 21 μl 2.5mM L-tyrosine. SP6:mCherry (7 μl) was used as a positive control and the mixture of cell-free components with water instead of any template DNA was used as the blank control. Unfortunately, no GFP-like signal was observed after overnight incubation (Fig. 10).
Enzyme activity assays
The absence of any detectable product might be a result of low measurement sensitivity or the lack of enzyme activity in the cell-free environment. In order to test whether the synthesized proteins possess the expected enzyme activity, we are working on monitoring the metabolites consumption and production in the cell-free reaction solutions by liquid chromatography-mass spectrometry (LC-MS). To start with, we focus on detecting the activity of CYP76AD1/6 by applying a high concentration of L-tyrosine (1.3mM) and monitor the production of L-DOPA. At the moment, we are optimising the equipment settings in order to separate L-tyrosine and L-DOPA on the liquid chromatography.
Conclusions and DiscussionUp to this point, we have tested the expression of various proteins in microdroplets with the combination of a commercial cell-free protein expression system and microfluidic devices. Betalain biosynthetic enzymes were also successfully coexpressed in microdroplets and confirmed by fluorescent protein fusions. However, simply coexpressing two enzymes from the betalain biosynthetic pathway in the cell-free protein expression solution was not sufficient to drive detectable conversion from tyrosine to either betanidin or betaxanthin no matter whether extra tyrosine was added.
The first step in the betalain biosynthetic pathway is the hydroxylation of L-tyrosine to L-DOPA, which is conducted by several different cytochromes P450 and also plays a vital role in the biosynthesis of dopamine. Cytochromes P450 are membrane-associated proteins and require heme cofactors to be functional. The production of active eukaryotic cytochromes P450 in Escherichia coli has proved to be very difficult, partially because of the lack of membrane structures and cofactors. So optimisation to the buffer conditions of the cell-free protein expression system is needed to produce active CYP76AD1/6 in microdroplets. Possible solutions might be the addition of membrane fractions/liposomes into the cell-free protein expression system, truncation of the N-terminal signal peptide from CYP76AD1/6, or even making microdroplets consisting of lipid bilayers to help the anchor of P450.
The mimic of betalain biosynthetic pathway in microdroplets provides a good platform to explore the possibility of using microdroplets in a large range of research, from the fundamental study of primary/secondary metabolic pathways to the bioproduction of high-value chemicals.
Future plansUp to now, the bottlenecks are the limitation of measurement sensitivity and the activity of in vitro expressed enzymes. In order to overcome these, we aim at using LC-MS as the standard technique to monitor reactions in the cell-free solutions or microdroplets in our future work. Future plans include:
1. Optimisation of the LC-MS conditions to separate L-tyrosine, L-DOPA and betalamic acid.
2. Measurement of CYP76AD1/6 activities and optimisation of cell-free conditions for better enzyme activities.
3. Measurement of DODA activities.
4. Combination of two enzymes in the cell-free environment and measurement of betalain productions.
5. Explore the possibility to control the metabolite flow between microdroplets in order to mimic multicellular pathways.



Comments
Please log in or sign up to comment.