We were inspired to take an inexpensive RepRap 3D printer (an open source 3D printer capable of printing a kit of itself) and convert it for bioprinting applications. Current commercial bioprinters are in the region of tens to hundreds of thousands of dollars, and we wanted to see if we could make a great one for a fraction of the cost. We were inspired by the 2018 paper by Kira Pusch et al. as a starting point.
For our project we'll start with an open source Creality Ender 5 3D printer, and convert it into a bioprinter, capable of printing bioinks. We document the process of converting the printer, and creating some example bioinks here.
Our long term goal is to use the converted printer, and ink chemistry to print coral.
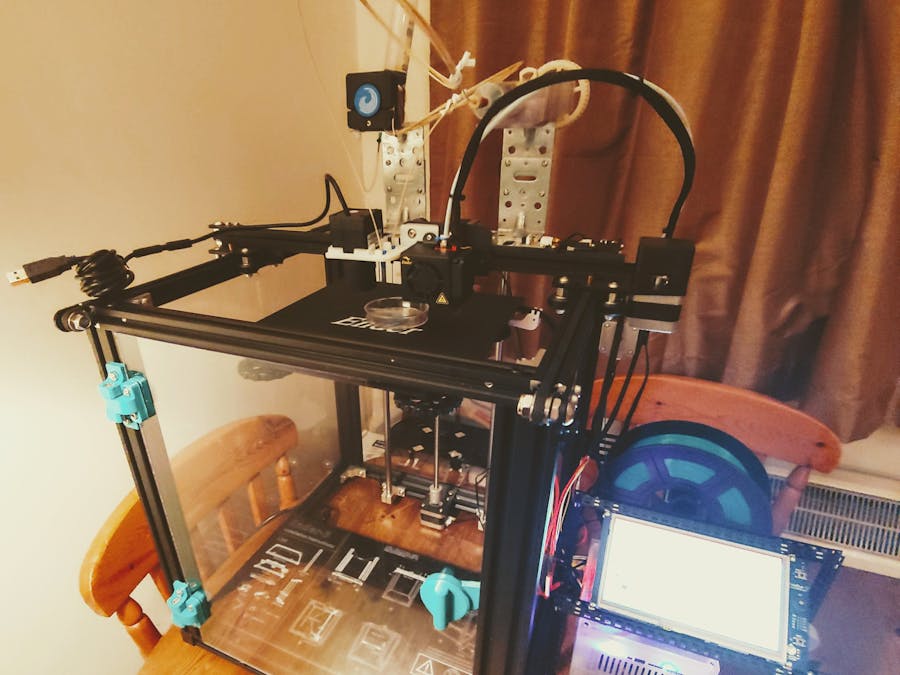
Assembling the Creality Ender 5The Ender 5 needs some assembly on arrival. Fortunately the process is very straightforward with the instruction manual provided. Below is also a video from Creality that documents the process, and a photo of our assembled Ender 5
Constructing the syringe pump extruderThe extruder is what drives the bionk material to the nozzle. We want to replace the standard PLA filament extruder of the Ender 5 with a syringe pump extruder. We followed the instruction video provided by Kira Pusch et al to build their Large Volume Extruder (LVE).
Our build steps are shown below:
Our version of the Large Volume Extruder (LVE):
In our case we used silicone tubing to connect syringe extruder to the nozzle. The tubing is not very stiff, and we tied it to some PLA filament to keep it in place.
Alternative: peristaltic pump extruderOne issue with the LVE is its large footprint. This would be more of a problem when we're printing with multiple nozzles (and so multiple extruders). To see if we could get the size down, we investigated using a peristaltic pump instead of a syringe pump. We found an inexpensive one, compatible with the Ender 5's Nema 42-40 extruder stepper motor, on Banggood.
In principle the only hardware modifications necessary will be attaching one end of the peristaltic pump to the syringe with some silicone tubing, and the other end to the nozzle of the printer again with tubing. The Ender 5's firmware will also need modifying to set the correct print speed for the pump (described in "Bioprinter conversion process" section).
It turns out the peristaltic extruder works quite well (video below). One issue was the hole in the pump for the Creality Ender 5 stepper motor shaft was too small. Using an M5*20 bolt and a hammer the hole was increased in size to accommodate the stepper motor shaft.
Nozzle replacementThe process to replace the nozzle involves removing the Ender 5's print head from the assembly. This is quite straightforward and shown in the pictures below. Remember to detach the wires too!!!
After that a syringe nozzle is attached to the assembly.
In subsequent stages of the project we left the existing print head on the printer, and added a bracket to hold our syringe needles. This way we would continue to 3D print components we needed for our project without having to continually take off the syringe needle print head and replace it with the PLA filament head.
Bacterial bioink literature reviewCombining 3D printing technology and living organisms is a relatively new field, and though the number of papers on 3D bioprinting is steadily increasing, not all organisms (including corals) have been covered. Our first attempt to 3D print a living organism will thus be conducted with non-pathogenic bacteria, a well studied and characterised organism commonly used in molecular biology and synthetic biology. We identified two main approaches to 3D print bacteria by doing a survey of the current literature. The first approach embeds bacteria in a hydrogel that provides an environment with a high water content to allow the exchange of nutrients and waste products (https://advances.sciencemag.org/content/3/12/eaao6804). The authors named their functional living ink 'Flink', which is composed of hyaluronic acid (HA), κ-carrageenan (κ-CA), and fumed silica (FS) in LB media. Changing the concentration of these constituants affects the viscosity and elastic properties of the ink and the authors show that a 1:1:1 ratio is the most sturdy hydrogel. Exchanging HA with a chemically modified glycidyl methacrylate HA (GMHA) to produce a 'Flink-GMHA' bioink further allows the hydrogel to be cross-linked after UV exposure (365nm, 60 secondsm 90mW).
The second approach exploits the interaction of sodium alginate and calcium chloride, which create a gel when in contact (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5525104/). Here, the authors develop a simple bioink composed of LB medium supplemented with 5% (w/v) sodium alginate, which is extruded on an agar petri dish which has 500uL of calcium chloride (1M) equally distributed on its surface. Unlike sodium (Na), Ca2+ is a divalent cation which can form two chemical links. Thus, when sodium alginate is in contact with calcium chloride, and the calcium ions replaces the sodium ions in the alginate solution, a gel is formed by cross-linking. These authors optimise the concentration of both ingredients and show that their 3D-printed bacteria survive and maintain their metabolic activity for at least 48h.
Because of the wide availability of sodium chloride and calcium alginate, which are used for cooking applications, its simple bioink preparation and the lack of necessity of a UV light, we decided to go forward with the latter approach for 3D printing of bacteria.
Bacterial bioink preparationIn order to visualise the bacteria in the gel, we use an E.coli strain which was transformed with a red-fluorescent protein expressing plasmid (http://parts.igem.org/Part:BBa_J04450:Design). This chromoprotein stains the bacteria slightly red under visible light and fluoresces red under 488nm or 532nm fluorescence. The bacteria transformation is accomplished by a member of the Bio Makespace (BMS) laboratory as a positive control for an unrelated experiment. Below is a step by step protocol for the formation of the bacteria-bioink.
1. Culture BBx-JO4550 transformed bacteria overnight in LB media supplemented with 25ug/mL kanamycin in a shaker set at 250rpm, 37°C. Prepare 10mL of bacterial culture for a 10mL bioink.
2. Centrifuge overnight culture at 1500rpm for 5 minutes and discard supernatant. Re-suspend bacteria in freshly prepared 5mL LB media supplemented with 25ug/mL kanamycin.
3. Prepare a 5% (w/v) sodium alginate solution in LB media, by weighing out 0.5g of sodium alginate and dissolving it at room temperature with 5mL of LB media.
4. Supplement the freshly prepared 5mL bacteria culture with 5mL of 5% (w/v) sodium alginate solution. This consists of the bioink and can be transferred to the printing syringe.
5. Spread 500uL of Calcium chloride (1M) on a pre-made agar plate and let airdry 5 minutes.
The bioink and the printing surface are now ready.
Bioprinter software conversion processPusch et al. list the G-Code Modifications necessary for bioprinting. The middle command stops the needle from leaving the petri dish print area (and possibly dragging the petri dish with it). We plan to make some changes to this code to better suit our needs.
M302; Enable cold-extrusion
G92 X73.5 Y70.5 Z0.00 E0.00 ; Tell the printer that the nozzle is homed and at a height of zero in Z to enable printing to begin at the manually-selected point. The X and Y values used correspond to one half the length of the X and Y build dimensions of the printer platform; the values used here are for a PrintrBot Simple Metal.
T0; Set the nozzle temperature to zero.One key step is to specify the correct print speed. To do this we need to modify the Ender 3 firmware. The video below describes how to modify Ender 5's firmware by first loading a bootloader to the Ender-5 so firmware code can then be sent to it via USB.
BioprintingTo bioprint we can use existing slicing software such as Cura or PrusaSlicer to generate the G-code files to print. Cura offers a nicer interface, but is limited in the number of extruders it can generate G-code for, while PrusaSlicer works with more than 2 extruders. Ensure the code generated has the G-code modifications described in the previous section.
We proceeded to use PrusaSlicer for our project.
Extending to multiple extruders - Duet+Duex5 Motherboard ReplacementTo extend the Ender 5 to support multiple extruders/nozzles in order to print more complex biological structures, we need to replace its motherboard with a DuetWifi, which is also attached to a Duex5 - which extends the DuetWifi to support up to 5 extruders. The process is described in the videos below:
We also found the following links very helpful:
1. Duet board connection setup
3. Wiring an Ender 3 with a Duet motherboard (Enders 3 & 5 are almost the same)
4. Configuring your Ender 3 Duet (Our Ender 5 settings are described in the attached code file)
We also 3D printed a case for our Duet Wifi and Duex 5, from the thingiverse thing here.
Retractable nozzle designOne issue with having multiple extruders is needing multiple needles. While one needle is printing the other needle will snag against the petri dish, displacing it and ruining the print.
Our plan to fix this was to use a linear solenoid actuator which shifts a needle down when it's printing and up when it isn't.
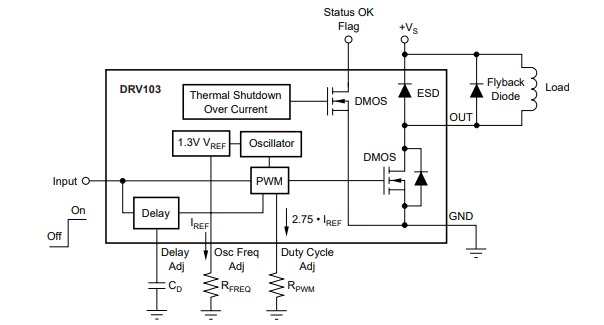
The Duex5 motherboard allows for connecting solenoids to the fan outputs, and switching them up and down via the printer's G-Code during a print. However, there is one big problem - the solenoids draw a lot of current when on, and they get unacceptably hot.
The current needed to activate the solenoid is a lot higher than the current needed to hold it in position while printing. Our solution therefore is to fire the solenoid with a large initial current, and then drop the current while holding the solenoid in place when printing.
Luckily someone online had already described the process of how this can be achieved, (see Overthinking solenoid control ), and we used this blog post as our inspiration to develop circuitry for powering the solenoids.
We have attached the circuit diagram developed at the bottom.
To control the temperature and humidity of the print bed, we built an enclosure around the printer. The below video describes how to build a temperature controlled enclosure for printing specialist materials.
Note: the width given for the front door in the video is 40mm too wide!!
Important note: The side panels should be 408 mm high not 410 so the don't rub on the timing belts, which ruins prints, degrades the belts and makes an unpleasant noise
We broadly followed the steps outlined in part 1, but used these hinges which have a metal rod (the hinge in the video was problematic to print and assemble with PLA filament, as the PLA rod kept snapping), and used the latch shown in part 2.
Version 1 - Needles and Camera
The first version of our print head provided space for two needles, two solenoids and a camera. We've attached the STL for this print head to the project. The camera would help us monitor our prints. It performs acceptably at 100-150 microns, and could be switched out depending on the resolution of what's being printed.
We had anticipated using smaller solenoids, and the space left on the print head for them was smaller. Unfortunately they didn't provide enough force to retract the needles, and we had to replace them with heavier solenoids. To do this we sacrificed the camera.
So we have two print head versions, depending on the application.
Version 2 - Solenoids and needles
Version 2 allows for two solenoids each extending and retracting a needle, but without space for a camera on the print head itself. The assembly allows for multiple needle gauges with a small change to a single piece rather than the whole assembly. The syringe is fed through the inner cylinder which is connected to the solenoid by an M3 bolt. when the solenoid moves, the inner cylinder, and so the needle, also moves. Our STL files are attached.
Version 3 - Servos and needles
We found the solenoids heavy for the print head, and in addition they proved less flexible for different printing situations. It was decided to switch to servos:
Bertie the Bioprinter's final assembly & printingFinally we assembled all the pieces, including adding brackets to hold our syringe pumps, to give our finished prototype, named Bertie:
For printing, we're using the PrusaSlicer and the following links have helped us for printing with multiple extruders . We're currently still refining the print settings to get good quality prints.
1. PrusaSlicer settings for the Ender 3 (pretty close to our ender 5)
2. Printing with multiple extruders using Slic3r (PrusaSlicer is a fork of Slic3r)
In face of the challenges encountered while venturing into bacteria and coral printing (described below), an alternate living organism was selected which could be cultured in the household (remove the requirement of transporting and/or storing the 3D printing elsewhere), would be relatively cheap and easy to maintain, survive as single cell organism smaller than 0.5 mm and have a practical application. Thus came our interest in phytoplankton, which are spherical algae cells growing as single cells of 2 - 3 um (see picture). Because of its tolerance to a broad salinity range and its potential for genetic manipulation, we were particularly interested in the genus Nannochloropsis with 6 known species living in marine, fresh or brackish water. Nannochloropsis is currently used as a feed in aquaculture of fish larvae or corals and also used for human consumption. They are suitable for industrial applications because they can accumulate high levels of polyunsaturated fatty acids, they are transfectable and some strains are capable of homologous recombination, all of which makes them suitable for genetic engineering. A recent example of their utility is the production of biofuel. By combining 3D printing and genetic manipulation, we can harnest the potential of algae for biotechnology applications.
Algae Culture
A Nannochloropsis phytoplankton culture was prepared according to the instructions of the culture kit provider, as detailed below.
1. Two liters of water was boiled and cooled down to room temperature.
2. The water was transferred to a sterile container and supplemented with 250mL of starting culture and 3mL of nutrient Guillards F/2 (all provided in the kit).
3. The Nannoclhoropsis were then cultured for 14 days with 16h of UV light per day and continuous pumping to prevent the cells of settling at the bottom.
4. On day 14, the culture was supplemented with 1.7L of water, previously boiled and cooled down to room temperature. The culture should become lighter in colour.
5. The Nannochloropsis were then cultured for further 7 days with 16h of UV light per day and continuous pumping. The colour should darken by day 7.
6. After 7 days, the algae was harvested by transferring approximately 70% of the liquid into a separate sterile jar. Each harvest can be stored for up to a week at +4C, with inversion of the jar every few days to prevent settlement of the algae at the bottom which would lead to rotting. The colour should remain stable if inverted often or otherwise concentrated at the bottom of the jar.
7. The remaining 30% of the algae will be supplemented with 1.5mL of nutrient Guillards F/2 and another 1.7mL of water, previously boiled and cooled down to room temperature. The colour should be lighter.
8. Repeat steps 5.-7. for a maximum of 3 months.
1. The algae should be left to settle at the bottom of the jar 24h before starting the bioink.
2. Remove superfluous liquid from the culture with pasteur pipettes provided in the kit to concentrate the cells.
3. Prepare a 5% (w/v) sodium alginate solution in distilled water, by weighing out 0.5g of sodium alginate and dissolving it in 5mL of concentrated algae culture.
4. Right before printing, add equal volumes of concentrated algae culture and 5% sodium alginate solution. This makes up the bioink and can be transferred to the syring..
5. Spread 500uL of Calcium chloride (1M) on a petri and let airdry 5 minutes.
The bioink and the printing surface are now ready for 3D printing.
Longer term goal - printing coral (aka bioprinting in the lab)Coral bioink preparation proposal
The proof of concept approach described above to print bacteria and algae will be adapted to meet the needs of coral, namely transparency to enable photosynthesis to be maximised as well as sufficient porosity to enable water circulation. Transparency being impacted by porosity, different percentage hydrogels will be tested to identify a sweet spot where optimal porosity and mechanical stability are attained. Concentration of coral organisms within the hydrogel will also be optimised to maximise coral growth.
Corals will be printed in a dry, humid environment at the optimal temperature for our chosen coral and subsequently transferred into aquariums for continued growth.
Bacteria and coral bioink current status
We have successfully created a hydrogel by dispensing a solution of 5% sodium alginate onto a surface covered by 1M calcium chloride. However, we encountered two problems detailed below which prevented us from further applying this approach to bacteria and corals. These challenges are by no means fully inhibitory, we just didn't have the resources or the necessary time to address them. We propose below some solutions to overcome them.
1. Bacteria
Culturing genetically modified bacteria such as the BBx-JO4550 transformed bacteria we were hoping to use as a proof of concept requires special permission. Fortunately, we managed to get our project approved at the Cambridge Biomakespace, which then allows us to print transformed bacteria with the calcium chloride/sodium alginate method and our 3D bioprinter. Since we were working extensively on the hardware of the 3D bioprinter and we only made it sturdier by the end of the project, it was in no state to be traveled back and forth the Biomakespace quarters in order to optimise the bioink. The facility also didn't have the space to store our printer. Now that towards the end of the project the printer is sturdier and is also accompanied by a traveling trunk for protection, we believe it will be easier to bring it more frequently to the Biomakespace to work on developing a bioink for bacteria.
2. Corals
Upon consulting with some experts in the field of corals, we've realised that printing corals is out of the scope of this project, especially considering the time line and the budget. Two criteria were originally selected in order to narrow our coral selection from the fewer than 1000 species: 1) individual polyps must have a diameter smaller than 0.5 mm and 2) corals must resist the process of fragmentation to obtain individual polyps. These criteria significantly reduced our selection to the family of small poly stony (SPS) corals, notably the Montipora genus. Unfortunately, we have found some discrepancy in the literature and various expert opinions on whether or not the corals can survive as individual polyps and how much time it would take for them to recover. Furthermore, all the SPS corals are notoriously difficult to culture as they require a large tank, a pump, specific UV lighting and daily nutrient checks. Because of the uncertainty behind the survival of corals as individual polyps and because none of us have previous experience with culturing corals, we could not justify the high upfront cost of the coral culture equipment. Although these roadblocks stopped us from pursuing this avenue, they should not prevent others from trying to 3D bioprint corals. Someone who has more experience in coral culture would be more informed in the choice of coral species and in keeping the difficult corals alive in an aquarium. Importantly, someone with enough longitudinal time and the necessary aquaculture equipment would be most suitable for this research. The output of this research may be vital to the world-wide effort for wild coral preservation.















Comments
Please log in or sign up to comment.